You are in a doctor’s waiting room. A few people who appear to be in distress are sitting on the chairs that line the walls. When you turn around, you find the door leading outside to be closed. You think about asking the receptionist if you can skip the queue, but wait- this isn’t real. You’re actually in the office of a clinical psychologist wearing a virtual reality (VR) headset and wielding a motion-tracked controller in each hand. If you tried to explore your surroundings, you’d run into one of the very real office walls – or worse, your doctor’s computer system.
This setting is one of the numerous scenarios that comprise gameChange, a virtual reality system developed by Dr. Daniel Freeman and his colleagues at the University of Oxford to treat psychosis using a form of medical technology known as digital therapeutics or DTx.
A scene from the gameChange VR system simulating a doctor’s waiting area
Source: gameChange
What is digital therapeutics (DTx)?
Digital therapeutics is defined by the Digital Therapeutics Alliance as “evidence-based medical interventions that use clinically evaluated software to treat, manage, and prevent a wide range of diseases and disorders.” DTx is a form of software-as-a-medical-device (SaMD), that uses software interventions to bring about behavioral changes in patients, to help treat their conditions.
It’s a subset of digital health, and a form of digital medicine, that can be used to maximize patient care and health outcomes through independent use or in conjunction with pharmaceuticals.
Digital therapeutics: A subset of digital health
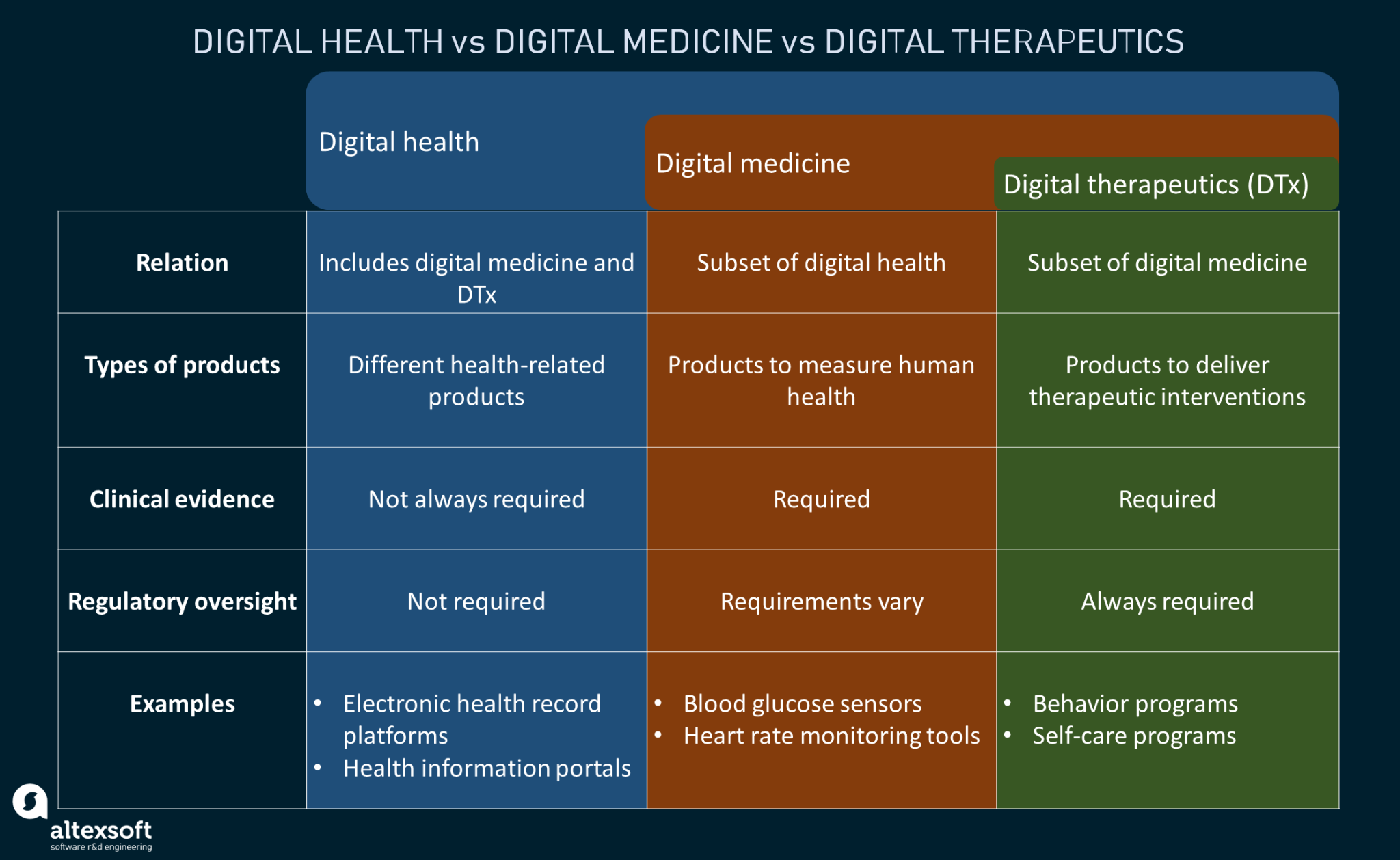
DTx is frequently used as a preventive measure for people at risk of acquiring more serious illnesses. A patient with prediabetes, for example, may be administered digital therapeutics as a way to improve their diet and behavior, which could otherwise lead to a diabetes diagnosis.
They can also be used to treat pre-existing conditions. A patient with type II diabetes, for instance, can use digital therapeutics to better manage the disease, using IoT-enabled insulin pumps and other health monitoring systems. This form of digital behavior-monitoring treatment not only ensures medication adherence and healthy lifestyle changes, but it ultimately reduces the need for more follow-up checkups and relapses, which traditional treatments pose a risk of.
The rise of digital therapeutics based treatments in healthcare
In the post-COVID era, the global healthcare system is nearing its tipping point, with the dual challenge of reducing healthcare costs, while also increasing capacity to treat patients. Digital technologies, particularly DTx, are providing a solution to these issues, leading to their widespread adoption.
According to Saurabh Gupta, Innovation Research and Strategy expert at Netscribes, COVID-19 has definitely proved to be an inflection point in the development, regulation, and implementation of prescription DTx applications across a variety of therapies, specifically mental health, and chronic ailments. It has brought the industry stakeholders (technology developers, regulating policymakers, physicians, payers, and insurers) to relook and reform the delivery outlook of healthcare services.
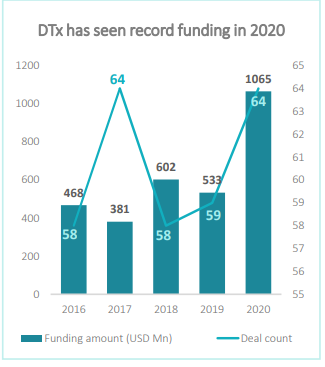
With the growing amount of positive evidence and advantages, DTx has seen accelerated adoption in recent years. The market witnessed a record funding year in 2020, receiving USD 1.07B over 64 deals.
Four key growth drivers in the digital therapeutics market
Here’s a look at the growth drivers that are driving the adoption of DTx in healthcare.
1. Increased need for virtual care, especially for chronic conditions
The pandemic has caused an increase in virtual care usage in the remote and home healthcare segments across the world. As DTx interventions are typically delivered via smartphones or tablets, there are few technological barriers to their implementation and scalability. In addition, the need to monitor the health of vast rural populations with chronic conditions like diabetes, cancer, and heart disease is also a driving force for the adoption of DTx.
In the past year, Welldoc’s DTx-powered diabetes management solution, BlueStar, saw an 80% and 60% increase in its blood glucose and blood pressure recording entries, respectively.
2. Growing clinical evidence of DTx’s effectiveness
DTx products are subject to rigorous patient-centered core principles, an industry code of ethics, and product development best practices. Unlike digital health and wellness apps, DTx products must publish clinical trial results in peer-reviewed journals and meet industry requirements. As a result, there’s a lot of readily available, credible evidence supporting DTx product claims and outcomes. Currently, there are more than 600 peer-reviewed efficacy and effectiveness studies for digital health as a medical intervention.
3. Better regulatory and clinical pathways
In early 2020, COVID-19 increased the demand for effective virtual health care interventions. The resulting increase in the use of DTx and remote healthcare led to the expansion of clinical pathways. For instance, the US FDA has permitted the marketing of the first game-based digital therapeutic to treat children with ADHD. The FDA has also been working on a precertification approach in which individual DTx companies, rather than specific interventions, can receive approval based on the quality of their development process.
While US Medicare does not currently cover DTx products, a Senatorial bill (S.3532-Prescription Digital Therapeutics to Support Recovery Act) would amend the Social Security Act to include prescription digital therapeutics for mental health and substance use disorders under Medicare and Medicaid.
4. Prevalence of personal devices such as smartphones
The increased use of personal technological devices in healthcare, such as smartphones, laptops, and tablets, has greatly aided in the adoption of DTx treatments. By delivering treatments or therapies via smartphones or tablets, DTx is helping increase patient access to the safety and effectiveness of treatments, presenting information in different languages, providing relevant information about diseases and treatments, and expanding physicians’ ability to care for patients. Furthermore, such a mode of care delivery, which can be easily implemented at home, eliminates any stigma associated with traditional therapies.
Major market trends in digital therapeutics
1. Telehealth players are leveraging DTx platforms to expand capabilities
With the pandemic significantly increasing the adoption of telehealth and virtual care, telehealth providers are looking to DTx platforms to boost the value of their services. For instance, Healthagen, Aetna’s telehealth business unit inside CVS Health, has collaborated with Welldoc and LifeScan Inc. to assess the use and potential health benefits of OneTouch Reveal® Plus. It is a coaching app for mobile and web that employs digital therapeutics to treat adults with type 2 diabetes. More than 10,000 Aetna members were invited to test the app’s efficacy.
Meanwhile, Amwell, a telehealth giant, has recently acquired SilverCloud Health, a DTx-based mental health platform. This acquisition will allow Amwell to deliver care in between one-time virtual visits, by using interactive DTx treatments. It also brings SilverCloud’s extensive list of 300+ healthcare partners to Amwell, including Kaiser Permanente, Optum, Providence Health, and the UK’s National Health Service, into the fold—partnerships that it can foreseeably leverage down the road to upsell its other virtual care products.
2. Diabetes and mental health are the top therapeutic areas embracing DTx
Healthcare firms have been investigating how DTx can be used directly or indirectly for the treatment of diabetes patients and patients with mental disorders. Studies have shown that by using DTx in the treatment of diabetes, patients have seen behavioral changes that are projected to prevent 60% of individuals with prediabetes from acquiring Type-2 diabetes.
Pharma firms and DTx firms have also been partnering to further develop solutions for these conditions. Pear Therapeutics, for example, teamed with Sandoz, a Novartis business, to promote the introduction of reSET, a treatment for substance abuse disorder, which is its first FDA-approved prescription DTx.
Meanwhile, Bayer has signed a licensing arrangement with One Drop to use the company’s diabetes management platform, and Sanofi has teamed up with Happify Health to develop a prescription DTx for depression treatment in people with multiple sclerosis.
3. North America is currently the largest and fastest-growing DTx market
North America, which includes the US and Canada, is the largest market for digital therapeutics. Market growth in this region is driven by factors such as the influx of new startups, increased investments in DTx, advancements in the reimbursement structure, and government initiatives to encourage technological advancements.
With the FDA revamping its regulatory processes to encompass DTx products and solutions, the US has emerged at the forefront of this industry, leading the growth of DTx not only in North America, but globally. The US DTx market was valued at USD 1.16B in 2019 and is expected to reach USD 5.08B by 2027 with a CAGR of 19.2%, making it the largest DTx market.
In the US, over 34.2M people have diabetes, constituting around 10.5% of the country’s population, and healthcare costs for these patients are 2.3 times more than non-diabetic individuals. This has resulted in the increased adoption of DTx-based treatments, which not only act as a preventive measure for pre-diabetics but also help those who have the illness to manage it better and in a more cost-effective way.
For instance, Blue Mesa Health, a digital health startup specializing in chronic diseases, offers remote and personalized DTx-based diabetes prevention programs to patients based on the Centers for Disease Control and Prevention (CDC)’s National Diabetes Prevention Program. Meanwhile, Virta, another DTx-focused diabetes treatment firm, pushes for Type-2 diabetes reversal while also attempting to lessen the severity of chronic diseases caused by diabetes, such as liver inflammation.
Get in touch with us for a detailed analysis of the digital therapeutics market.
What does the future look like for DTx?
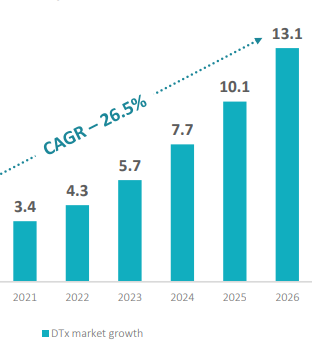
In the long run, DTx products have the potential to greatly improve the existing healthcare system. As a result, the global DTx market is positioned for a bright future, with a CAGR of 26.5% expected to reach USD 13.1 billion by 2026.
The forecasted market growth of DTx (in USD B)
Meanwhile, to facilitate the adoption of DTx, the healthcare industry’s focus needs to be driven on the following:
- Integration of software-as-a-medical-device (SaMDs) into old workflows: Continuous advancements in DTx software are utilized to focus on chronic conditions, and incorporating this into traditional healthcare workflows can assist in reducing physicians’ time spent on recurring conditions and cases, since DTx can help patients manage their conditions better.
- Maintaining regular engagement and close collaboration with HCPs: Working with medical fraternities and health societies, as well as hosting webinars, periodic learning sessions, and revising educational content, can all assist DTx companies in refining their products and services and addressing any potential post-market complications.
- Electronic health record (EHR) interoperability: EHR interoperability will assist EHRs in managing the influx of data created by DTx products from several sources at the same time and will make it available to HCPs at their fingertips. It also ensures that data security and privacy standards are met, which is critical for all forms of digital medicine like DTx.
- Building a clinical evidence database: Building a robust and stringent clinical evidence base can aid DTx firms not only while they’re formulating therapies, but it can also help HCPs use them effectively once they’re commercialized. An efficient medical evidence base will also include protocol design and research design frameworks, which help while applying for regulatory approvals.
COVID-19 has shaped the way digital health technologies enhance healthcare providers’ ability to provide successful patient care. The future expansion and adoption of DTx, on the other hand, is heavily reliant on industry leaders and stakeholders understanding the value of these solutions and employing a collaborative approach to establishing unified frameworks and streamlined regulatory pathways.
“In a nutshell, currently and even in the future, the DTx adoption and growth is likely to remain a regional trend with the digital divide scenario(s) clearly visible in the picture. Countries wherein the stakeholders cohesively collaborate and transform processes, solutions, platforms, and delivery modes in the healthcare universe will see a much rapid adoption of DTx solutions, vis-à-vis other countries.”
– Saurabh Gupta, Innovation Research and Strategy, Netscribes
Netscribes provides healthcare firms with comprehensive market, customer, and technological insights to help them navigate industry changes. Contact us, to know more.






